About us
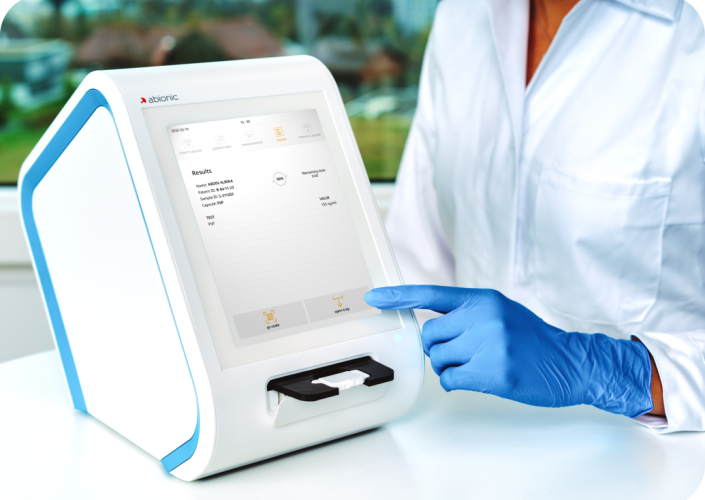
Abionic, an emerging medical diagnostics company focused on rapid early detection technologies, received 510(k) clearance for its PSP test from the U.S. Food and Drug Administration (FDA) to accelerate the Time-to-Detection of Sepsis in critical care and emergency settings. Already certified under the EU IVDR as of July 2022, this FDA clearance marks a pivotal moment for Abionic’s expansion into the U.S. market.

Our vision
Abionic’s “First-in-Class” Rapid Testing solutions will become the Gold Standard for near-patient Early Sepsis Detection across Hospital workflows.
Iwan Märki, CTO and co-founder of Abionic
Our certificates
At Abionic, regulatory compliance is at the heart of everything we do. Our certifications, including FDA 510(k) clearance, ISO 13485, CE Mark, and IVDR compliance, demonstrate our dedication to meeting the highest standards of quality, safety, and performance.
ISO Certificate
CE Certificate
IVDR QMS
Looking to join Abionic?
We always seek curious minds, bold thinkers, and dedicated professionals ready to make an impact.
